Spark Plasma Sintering (SPS)

The Spark Plasma Sintering (SPS) method is an effective technique for the compaction of powder materials. A main characteristic of this method is the direct heating of the pressing tool and/or the sample by pulsed direct electrical current with low voltage. This results in high heating rates and allows for short treatment times in order to obtain highly compacted sinter bodies. The material transport (e.g. by diffusion) occurring during the sintering process can also be used for performing chemical reactions. Especially the conditions during the SPS process allow the use of the method also as an alternative synthesis route for intermetallic compounds, of which, some can be obtained only with difficulties by other techniques.
The MPI for Chemical Physics of Solids was the first institute in where an SPS setup had been installed. Two SPS apparatus are available, one of them being installed inside an Argon-filled glove box, which make it very useful for the compaction and/or synthesis of air/moisture sensitive samples. Both machines allow for external forces up to 50 kN, direct current up to 1500 A and a voltage limit of 25 V with typical pulse length of 3 ms. Within the Dresden SPS facilities we have established close cooperations with the Fraunhofer IFAM and IKTS institutes. Currently, there are 5 SPS machines of different size and capabilities in these facilities.
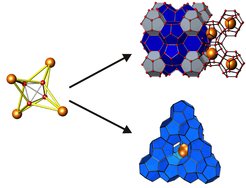
An example of a successful chemical synthesis in an SPS process are the clathrate phases K8-δSi46 (δ=0÷1.5), Rb6Si46, Rb11Si136, Cs8Si136, K8Sn44, Na2K6Si46, and Na8(Si,Ge)46. They were obtained via a solid-state electrochemical variant of the redox reaction by using binary or ternary alkali-metal Zintl phase precursors [1].
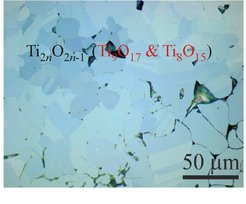
Also the single step synthesis of binary titanium oxides and substituted derivatives, starting from rutile and elemental titanium, shows the relevance of the diffusion controlled reaction during SPS experiments [2]. Based on our experience, several compounds were successfully synthesized, e.g. transition-metal oxides, binary and ternary borides, silicides, germanides.


