Starting from investigations of the chemical physics of rare-earth compounds, the corresponding actinide element systems (Th, U) promise considerable insight into unconventional electronic behavior. Due to a subtle interplay between the constituting elements with respect to their electronic configuration and their physical properties intermetallic compounds represent a wide research area of solid state physics and chemistry.
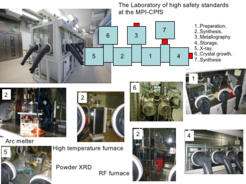
For this purpose alloys, compounds as well as crystals are synthesized and are subjected to chemical and physical characterization. Since toxic metals are used, special safety precautions are necessary. Inhalation of metal dusts and their escape into the environment has to be prevented. Therefore, all experiments with a latent danger of exposition are performed in a laboratory of high safety standards in closed set-ups under an atmosphere of argon gas. Each glove-box module is dedicated to specific scientific experiments: preparation, synthesis, depot for samples and chemicals, X-ray and metallographic analysis, and crystal growth.
As an example we present the intermetallic compound ThPt2.
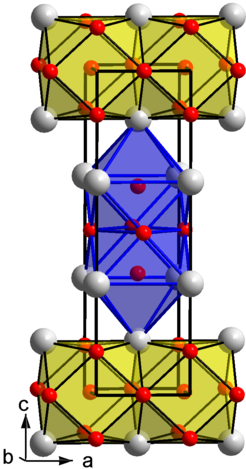
White spheres: Th; red spheres: Pt
ThPt2 crystallizes with unique type of structure, which belongs to the group of the close packed tetragonal structures with Pearson symbol tI12. An analysis of the chemical bonding by the electron density/electron localizability approach reveals formation of two-dimensional platinum polyanions being separated by the Th cations. Measurements of magnetic susceptibility, electrical resistivity and specific heat show ThPt2 to be diamagnetic with metallic type of conductivity in good agreement with the calculated electronic structure (N(EF) = 0.92 states eV-1 f.u-1).
A.Leithe-Jasper, R. Gumeniuk, W. Schnelle, U. Burkhardt, H. Borrmann, and Yu. Grin
IMR-ASRC 3rd REIMEI Intl. Workshop, Tokyo 2013

