The thermoelectric efficiency of materials is firstly characterized by a figure of merit, the so called ZT-value. In the formula
S stands for thermopower, s for electrical conductivity, k for the thermal conductivity and T is the temperature. To achieve a high figure of merit, materials should have high thermopower and electrical conductivity, but low thermal conductivity. Unfortunately for thermoelectric research, these properties are correlated by basic laws of physics and cannot be optimized independently.
Metallic conductors are not suited. They have in fact high electrical conductivity but low thermopower and high thermal conductivity which results in low ZT values.
Insulators show high thermopower and, in many cases, a favorably low thermal conductivity, but these properties are spoiled by the electrical conductivity close to zero.
Best ZT-values are obtained for semiconductors with sufficiently high electrical conductivity. Thermopower and electrical conductivity can be optimized by changing chemical composition. The lattice part of thermal conductivity is lowered, as a rule of thumb by heavy atoms and by complex crystal structures.
However, considering that a thermoelectric material separates a hot reservoir at T1 from a cold reservoir at T2, a high efficiency is required over the whole temperature range between these temperatures and not solely a high peak value at a certain temperature. Moreover, p- and n-type conductors, which are combined in the power generator, need to have similar thermal expansion coefficients and good mechanical properties too.
In the department of Chemical Metal Science at MPI CPfS, several kinds of systems are investigated, which have a potential to become thermoelectric materials. Examples are intermetallic clathrates and phases with FeGa3 crystal structure type.
Intermetallic Clathrates
The crystal structure of intermetallic clathrates is built up by a four-bonded framework which encloses large polyhedral cages. The framework is usually based on group 14 elements, while the cages are filled by electropositive metal atoms. In case of the so-called clathrate-I type, the crystal structure can be described by a packing of 20-atom and 24-atom cages.
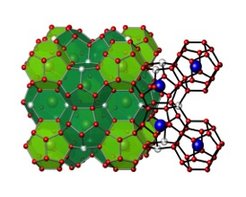
An example for a Clathrate-I with high thermoelectric efficiency is the phase Ba8Au5.33Ge40.67 with ZT ≈ 0.9 at 700°C.
Here, the framework is built up by Ge and Au atoms, the cages are filled by Ba atoms.
