Weak interactions under pressure: hp-CuBi and its analogues
Metallic materials are an essential part of everyday life since they serve in a manifold of functional tasks. The common metals are characterized by their pronounced affinity to form a broad variety of alloys and compounds. However, a remarkable exception are heavy main group metals such as bismuth which are located at the lower end of the periodic table. They exhibit a complete lack of this chemical reactivity when mixed with normal transition metals like cobalt or copper. This disadvantage limits their potential in applications although they exhibit often fascinating properties. One example for such an outstanding feature is their pronounced spin-orbit splitting caused by relativistic effects which substantially modify their electronic energies.
Scientists of the Max Planck Institute for Chemical Physics of Solids could overcome this obstacle and induce the formation of the compounds CuBi and earlier CoBi3 by performing chemical synthesis at extreme conditions. The selected parameter combination corresponds to that of regions within the upper mantle of the earth, i.e., a reaction pressure of about 50.000 atmospheres combined with a temperature of 450 °C. Analysis of the chemical bonding by advanced quantum chemical methods evidences that the phases exhibit weak but nevertheless strongly anisotropic chemical interactions. These interactions involve covalent multicenter bonding in strongly interconnected building units combined with pronouncedly weaker interactions of the lone electron pairs in the regions in between (see figure).
As both compounds exhibit unresisting electrical transport properties at low temperatures, a systematic comparison of superconducting and bonding properties of transition metal-bismuth phases was performed. The rather unexpected finding is that the transition temperature of heavier constituents is systematically higher than that of lighter elements - a result which is in contrast to the basic theory of superconductivity. Although the number of investigated superconducting compounds is not very large, the study reveals the importance of charge transfer and, thus, chemical bonding, which here overcompensates the effect of atomic mass for the transition temperature. More extensive studies will be necessary to verify whether this trend is based on a more general principle which lights on crucial characteristics of phonon-driven superconductivity.
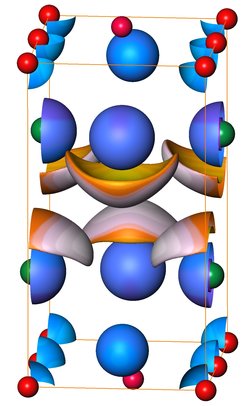
While normal metals like cobalt or copper exhibit a strong tendency for alloy formation, heavy main group metals like bismuth show a remarkable lack of reactivity. Scientists of the Max-Planck Institute for Chemical Physics of solids could overcome this obstacle by high-pressure synthesis yielding the compounds CuBi and earlier CoBi3. A systematic analysis reveals that the superconducting properties of transition metal-bismuth compounds violate a prediction of the standard theory of superconductivity.
US, JG / CPFS
