Chemical Bonding Analysis as a Guide for Preparation of New Compounds: The Case of VIrGe and HfPtGe.
So test therefore, who join forever, if bond to bond be found together! (after F. Schiller)
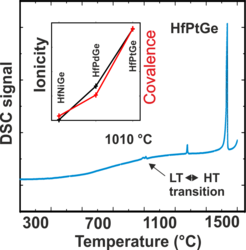
The versatility of electronic properties of the large class of MgAgAs-type compounds (half-Heusler phases) has moved them into the focus of material scientists. But for the equiatomic composition ABC there exist more than 40 competing structure types, and the question arises about which are the factors governing the choice of the MgAgAs-type of structure? Despite, and even because of the large abundance of computer time nowadays, Prof. Wigner’s admonition is still valid: “It is nice to know, that the computer understands the problem. But I would like to understand it too.” Along these lines, systematic research on quantum-mechanically based chemical bonding models in position space have led to the development of a conceptually simple quantification of covalence and ionicity for MgAgAs-type of compounds. The significance of this model has been tested to predict novel phases with this type of structure. Subsequent manufacturing of the six selected candidates yielded four compounds of the desired structure type, with VIrGe and HfPtGe yet unknown in the MgAgAs-type of structure.
FRW, YG / CPfS