Composite anionic lattices
The first new materials in this project were obtained using two different synthesis routes. First, by reacting alkaline-earth metal oxides with elemental transition metals and heavier chalcogens, i.e. sulfur, selenium, and tellurium, pure powders were achieved though solid-state reactions. Second, to synthesize single crystals, large enough for structural characterization, two different flux growth methods were used: A self-flux growth method and a flux growth in alkali-metal halide melts.
As all constituents are either moisture sensitive or might oxidize in air, all synthesis steps were performed under inert conditions, either in a glove box with controlled argon atmosphere or in evacuated, closed silica glass vessels.
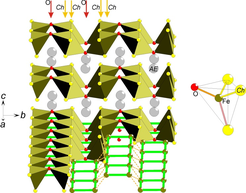
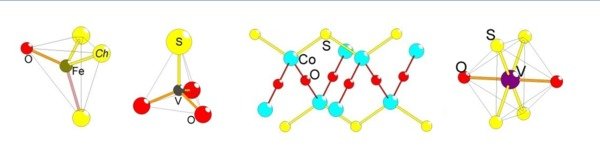
Figure 1. A perspective view of the BaFe2S2O structure with extended Fe sublattice to display the ladders. The local Fe coordination is displayed to the right.
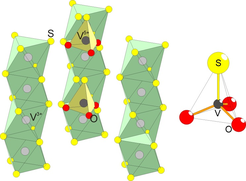
Figure 2. (left) The V-S-O lattice in Ba3V2S4O3, emphasizing the charge disproportionation order. (right) The polar VSO3 tetrahedron.
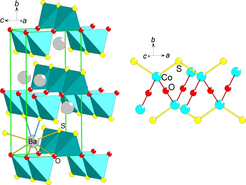
Figure 3. A perspective view of (left) the BaCoSO crystal structure and (right) the Co-S-O lattice.
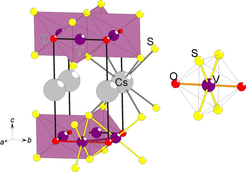
Figure 4. (left) The layered crystal structure of CsV2S2O and (right) the trans-octahedral VS4O2 coordination.



