
Osmates and novel transition metal oxides
The 5d transition metal compounds show very intriguing magnetic properties due to the presence of strong spin-orbit (SO) coupling [1-5] which competes with the on-site Coulomb repulsion and crystal-electric field (CEF) interaction. The balance of these interactions drives the physics and ground state properties. Here we started exploring the physical properties of the osmates. The Os valence can be 4+, 5+ and 6+, thereby giving possibilities for singlet magnetism to occur (5d4, Jeff=0), spin-only magnetism (5d3, S=3/2), and magnetism with orbital degrees of freedom (5d2, S=1/Jeff=1), assuming an octahedral-like coordination of the Os ions in an insulating compound. Osmates in the double perovskite structure were synthesized and when putting in non-magnetic ions on the B sites, we can nicely observe magnetic frustration phenomena for Os 5d3 [Paul2014] and 5d2 [Sarapulova2015].
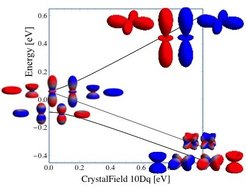
Energy level diagram of a single d electron with spin-orbit coupling as a function of the cubic crystal-field parameter 10Dq. The orbital plots show the electron charge density collored according the the z projection of the spin. On the left the j=3/2 and j=5/2 eigenstates in spherical symmetry are shown. On the right one can see the six t2g (low) and four eg (high) orbitals. The t2g orbitals split into a quarted (low) and doublet (high). At a filling of d4 the lowest quarted is filled and the material is non-magnetic.
The role of band formation is another, yet far less studied aspect in this class of materials. SO have Mazin-Valenti-Khomskii recently argued that the magnetism in Na2IrO3 can be better described in terms of molecular orbitals than in terms of atomic physics [6], i.e. strong inter-site hopping will quench the local physics. Here we will utilize Os4+ (5 d4) oxides for the investigation of the competition between SO coupling and band formation in 5 d materials. In a localized picture, the strong octahedral crystal field at the Os4+ site leads to a t2g4 configuration. This nine-fold degenerate state is split by spin-orbit coupling into a singlet ground state, with triplet and quintet excited states as shown in the Figure. The effective orbital moment l = 1 couples anti-parallel to the total spin S=1 to form a singlet with Jeff=0 in the ground state. The presence of a magnetic field or exchange field will induce magnetism in the system, due to the energetically nearby Jeff=1 state. Van Vleck-type excitations or superexchange stabilization of the Jeff=1, 2 states have been proposed as possible origin of the magnetic ground state because the singlet and triplet states are close in energy [6,7].
If the Os4+ ions in the double perovskite La2MgOsO6 (and La2ZnOsO6) can be considered as isolated ions, then we should expect to see the singlet magnetism as described above. However, in the limit that the hopping between the Os ions is dominating, we will end up with a band material and even a metal in which the magnetism may be given by the Pauli magnetism. This is probably the case in OsO2. Therefore, it is interesting to see whether for La2MgOsO6 (and La2ZnOsO6) how much of the local physics is conserved. A direct method to determine this is to measure the amount or orbital moment of the Os using XMCD.
- G. Cao et al., Phys. Rev. Lett. 112, 056402 (2014).
- M. Wakeshima et al., J. Alloys. Compounds, 287, 130-136 (1999).
- B. J. Kim et al., Phys. Rev. Lett. 101, 076402 (2008).
- B. J. Kim et al., Science 323, 1329 (2009).
- X. Liu et al., Phys. Rev. Lett. 109, 157401 (2012).
- I.I. Mazin Phys. Rev. Lett. 109, 197201 (2012).
- G. Khaliullin et al., Phys. Rev. Lett. 111, 197201 (2013).
- O. Nganba Meetei et al., Phys. Rev.B 91, 054412 (2015).
