Crystal chemistry of ternary noble metal borides
The inorganic structural chemistry of boron and its compounds can be characterized by a remarkable complexity which is due to the preferentially formed one-, two- and three-dimensional arrangements of covalently bonded boron atoms. This is mainly driven by the need of saturation of the valence requirements of the constituting electron-deficient boron atoms. The topological dimension of these aggregates can be correlated with the element-to-boron ratio. This relation has been particularly explored for compounds composed of metal atoms (transition elements, rare earth metals, actinides) and boron. There, depending on the metal content the boron substructure evolves from isolated boron atoms to boron pairs, fragments of chains and infinite chains followed by formation of two-dimensional frameworks. Further increase of the boron fraction gives rise to the formation of three-dimensional covalently bonded boron frameworks. Here the type and degree of connectivity of boron polyhedra as well as the occupation of the interstitial sites by metal atoms become the structure governing elements.
Joint research at the MPI-CPfS together with the Partnergroups in Moscow and Shanghai is focused on novel routes of synthesis, the relationship of the crystal/electronic structure to physical properties in such compounds as well as on the study of the chemical bonding situation in position space. Here we give a short overview of a selection of compounds which have been recently studied.
SrPd4B and BaPd4B: new type of crystal structure and physical properties
Two new intermetallic alkaline-earth palladium borides, SrPd4B and BaPd4B were synthesized and their physical properties were investigated. Their crystal structure is unique. The relationship of this structure type with the series of derivatives of the CaCu5 type is discussed. Calculated electronic band structures for palladium, Pd3B, SrPd5, SrPd4B and SrPd3B are compared. SrPd4B shows metallic behavior. Magnetic properties, electrical resistivity and specific heat capacity measurements reveal that the two compounds are diamagnetic metallic conductors with low electronic density of states, in agreement, with the electronic structure calculations.
![(Left) Boron-centered trigonal prisms in the CeCo3B2, CeCo4B and SrPd4B structure types: a) common fragments consisting of two edge sharing three-capped trigonal prisms [BT6R3] b) arrangement of the fragments [BT6R3] highlighted in a) and c) spatial arrangements of trigonal prisms [BT6] centered by boron atoms. (Right) Total and partial density of states for Pd, Pd3B (Fe3C type), Pd3B (fictitious AuCu3 type), SrPd3B, SrPd4B and SrPd5.](/2870696/original-1518441227.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjI4NzA2OTZ9--58025ffc576cd4cd3bcae2b3a2439d620122964f)
(Left) Boron-centered trigonal prisms in the CeCo3B2, CeCo4B and SrPd4B structure types: a) common fragments consisting of two edge sharing three-capped trigonal prisms [BT6R3] b) arrangement of the fragments [BT6R3] highlighted in a) and c) spatial arrangements of trigonal prisms [BT6] centered by boron atoms.
(Right) Total and partial density of states for Pd, Pd3B (Fe3C type), Pd3B (fictitious AuCu3 type), SrPd3B, SrPd4B and SrPd5.
(Left) Boron-centered trigonal prisms in the CeCo3B2, CeCo4B and SrPd4B structure types: a) common fragments consisting of two edge sharing three-capped trigonal prisms [BT6R3] b) arrangement of the fragments [BT6R3] highlighted in a) and c) spatial arrangements of trigonal prisms [BT6] centered by boron atoms. (Right) Total and partial density of states for Pd, Pd3B (Fe3C type), Pd3B (fictitious AuCu3 type), SrPd3B, SrPd4B and SrPd5.
R. Gumeniuk, M. Schmitt, W. Schnelle, U. Burkhardt, H. Rosner, and A. Leithe-Jasper; Z. Anorg. Allg. Chem.; 636, 954–961 (2010)
Boron induced change of the Eu valence state in EuPd3Bx (0≤x≤0.53): a theoretical and experimental study
In a joint theoretical and experimental study we investigated a large series of EuPd3Bx and GdPd3Bx compounds. Characterization by x-ray diffraction, metallography, energy-, and wavelength-dispersive x-ray spectroscopy as well as chemical analysis determine an existence range of EuPd3Bx up to x ≤ 0.53 and x ≤ 0.42 for the GdPd3Bx compounds, respectively. Our density-functional-based electronic structure calculation predict a valence change in EuPd3Bx above xcDFT= 0.19 from a nonmagnetic Eu3+ state into a magnetic Eu2+ state which is reflected in a discontinuity of the lattice parameter. In contrast, the related Gd compounds with a stable Gd3+ state exhibit an almost linear behavior of the lattice parameter following Vegard’s law. Consistent with the calculations, the x-ray diffraction data show a kink in the lattice parameter for EuPd3Bx at xcXRD = 0.22. X-ray absorption spectroscopy measurements assign this kink to a transition into a heterogeneous mixed valence state for Eu with a critical B content xcXAS= 0.22. The observed change in the mean Eu valence from Eu3+ x ≤ 0.2 toward Eu2.5+ x ≤ 0.5 is supported by magnetic susceptibility and specific-heat data.
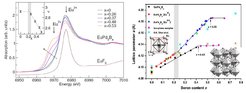
(Left) Eu LIII XAS spectra of EuPd3Bx for different B contents in comparison to the reference compound EuF3. The relation between contributions originating from Eu2+ and Eu3+ changes with increasing B content. The broken arrow points in direction of decreasing Eu valence increasing B content x. Inset: Development of the mean Eu valence as a function of the B content x. (Right) Experimentally observed variation in the primitive unit cell parameter a in REPd3Bx, RE= Eu, Gd, versus boron concentration x. (Right) Experimentally observed variation of lattice parameter a versus boron concentration x. In the left inset we see the parent CaTiO3 type of structure and in the right inset the Ti2Rh6B type of superstructure adopted for x > 0.35.
R. Gumeniuk, M. Schmitt, C. Loison, W. Carrillo-Cabrera, U. Burkhardt, G. Auffermann, M. Schmidt, W. Schnelle, C. Geibel, A. Leithe-Jasper, and H. Rosner; Phys. Rev. B 82, 235113 (2010
TM7TM′6B8 (TM = Ta, Nb; TM′ = Ru, Rh, Ir): new compounds with [B6] ring polyanions
The ternary boron compounds TM7TM′6B8 (TM = Ta, Nb; TM′ = Ru, Rh, Ir) were prepared by high-temperature thermal treatment of mixtures of the elements. An analysis of the chemical bonding by the electron density/electron localizability approach reveals formation of covalently bonded polyanions [B6] and [TM′6B2]. The cationic part of the structure contains separated TM cations. In agreement with the chemical bonding analysis and band structure calculations, all TM7TM′6B8 compounds are metallic Pauliparamagnets (TM′ = Ru, Rh) or diamagnets (TM′ = Ir).
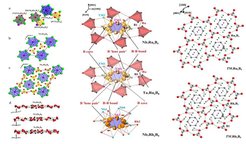
(Left) Crystal structures of TM7Ru6B8 and TM7Rh6B8: (a) coordination polyhedra of the TM, TM′, and boron atoms; (b, c) trigonal prisms around the boron atoms (blue, [BTM6]; yellow, [BTM′6]); (d) B–TM′ layers in the crystal structures of Nb7Ru6B8, Nb7Rh6B8, and Ta7Rh6B8 showing the evolution of puckering (Nb7Rh6B8) and disorder (Ta7Rh6B8). (Middle) Localization domains of ELI-D for Nb7Ru6B8 and Ta7Ru6B8 as well for Nb7Rh6B8 highlighting the bonding situation in the B6 rings (violet). Red polyhedral Ru–B units are shown for TM7Ru6B8 . (Right) Atomic interactions in TM7TM′6B8 compounds: gray lines connect the atoms in the polyanions, planar B6 rings, and 3D TM′6B2 species; red lines show the additional TM–Rh interactions in TM7Rh6B8 (TM, pink and green; TM′, red; boron, gray).
Ternary magnesium rhodium boride Mg2Rh1-xB6+2x with a modified Y2ReB6-type crystal structure
The new ternary magnesium rhodium boride Mg2Rh1-xB6+2x has been prepared by the reaction of the mixture of Mg powder, RhB, and crystalline boron in a Ta container sealed under argon. It represents a modified Y2ReB6 structure type with an unusual replacement of part of the Rh atoms by boron pairs located in the pentagonal channels parallel to the c axis. The pairs interconnect the neighboring planar boron nets into the 3D framework. The random distribution of the Rh atoms and boron pairs and the stabilizing effect of the boron pairs on the Y2ReB6 type structure motif are discussed using electronic band structure calculations and chemical bonding analysis in positional space.
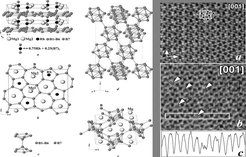
Crystal structure of Mg2Rh0.75B6.5: (a) alternation of the atomic layers perpendicular to [001]; (b) [001] projection of the structure with full occupancy of the Rh position; (c) interconnection of the planar nets by the additional boron atoms in the B7 position; (d) pentagonal-pyramidal units in the crystal structure of α-boron; (e) pentagonal-pyramidal units in the crystal structure of MgB4. [001] HREM image of Mg2Rh0.6B6.8 along [001]: (a) comparison of the experiment with the calculated image (inset), with unit cell indicated by a white rectangle and a scheme of the boron net; (b) point like defects (marked by arrows and with black rectangle) related to the preferential occupation of the positions by boron pairs; (c) strong contrast change along the region indicated with the white rectangle in (b).
![(Left) Boron-centered trigonal prisms in the CeCo3B2, CeCo4B and SrPd4B structure types: a) common fragments consisting of two edge sharing three-capped trigonal prisms [BT6R3] b) arrangement of the fragments [BT6R3] highlighted in a) and c) spatial arrangements of trigonal prisms [BT6] centered by boron atoms. (Right) Total and partial density of states for Pd, Pd3B (Fe3C type), Pd3B (fictitious AuCu3 type), SrPd3B, SrPd4B and SrPd5. (Left) Boron-centered trigonal prisms in the CeCo3B2, CeCo4B and SrPd4B structure types: a) common fragments consisting of two edge sharing three-capped trigonal prisms [BT6R3] b) arrangement of the fragments [BT6R3] highlighted in a) and c) spatial arrangements of trigonal prisms [BT6] centered by boron atoms. (Right) Total and partial density of states for Pd, Pd3B (Fe3C type), Pd3B (fictitious AuCu3 type), SrPd3B, SrPd4B and SrPd5.](/2870696/original-1518441227.jpg?t=eyJ3aWR0aCI6ODQ4LCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6Mjg3MDY5Nn0%3D--a20366c98515ae6691fbe2a0177a2b9f328cdc00)


