Chemical properties of intermetallic compounds
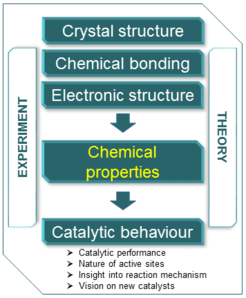
The research on intermetallic compounds (IMCs) includes a comprehensive insight into crystal and electronic structures, composition, formation route as well as atomic interactions between the parent elements in the particular crystal structure. These data form the necessary platform for understanding of their properties and vision of possible applications. Contrary to physical properties, which are extensively studied and are in the focus of many research groups and institutions, the chemical properties of IMCs remain scarcely presented and hidden in the literature.
Aiming to couple the fundamental interest and application feasibility, the chemical catalysis field came into our focus. On one hand, catalysis is a perfect playground to study the chemical function of IMCs under different reaction conditions. On another hand, intermetallic compounds is an intriguing class of materials for catalytic applications. The chemical properties of IMCs form the link between the chemical nature of the compound and its catalytic performance. The latter is governed by the state of the surface, which is originated from the chemical processes, occurring on the surface and in the bulk. Therefore, deep understanding of the chemical properties is obligatory for future development of new catalysts.
Reactivity of Ca-Ag compounds under ethylene epoxidation conditions
The prominent example of diverse chemical behaviour of IMCs was discovered during the investigation of Ca-Ag compounds as catalysts for ethylene epoxidation [1]. According to thermodynamics, all Ca-Ag compounds should be oxidized in the presence of oxygen. Nevertheless, even two neighbour compounds, namely CaAg2 [2] and CaAg [3], possess unique chemical properties under reaction conditions. Contrary to CaAg2 compound, which undergoes the oxidation towards elemental Ag and complex Ca-containing matrix (CaO, Ca(OH)2, CaCO3 etc.), the equiatomic CaAg remains stable in bulk over long-term experiment. The chemical bonding analysis and surface energy calculations clearly show the pronounced anisotropy of the atomic interactions in CaAg, which leads to the preferential cleavage of the material upon crushing. Furthermore, the preferred cleavage surface (010) is suitable for the formation of stable, ordered and dense CaO overlay. This overlay acts as passivation layer (similarly to aluminum passivation layer) and prevents bulk oxidation of the material. Since catalytic performance depends on the rates of concurrent chemical processes on the surface, the catalytic response is clearly different for these two materials.

[1]
Antonyshyn I., Sichevych O., Ormeci A., Burkhardt U., Rasim K., Titlbach S., Armbrüster M., Schunk S.A., Grin Yu. Ca-Ag compounds in ethylene epoxidation reaction. Sci. Technol. Adv. Mater. (2019). DOI: 10.1080/14686996.2019.1655664.
[2]
[3]

