New preparation routes for intermetallic compounds
Intermetallic phases often are prepared from the melt of the elements with a subsequent heat treatment, if necessary. By this, products are obtained, which usually are in thermodynamic equilibrium at least at reaction conditions. High-temperature phases being metastable at room-temperature are also accessible in this way, if they may be kinetically stabilized by rapid quenching. Analogously, high-pressure phases having been cooled down before the release of the pressure may be obtained in metastable state at ambient pressure. However, the preparation of intermetallic phases, which do not possess a thermodynamic stability field in the whole p-T-diagram at a given composition, takes more effort. A research focus of the Institute Chemical Metals Science lies on the development of accordingly suited preparation methods and on the preparation and characterization of new intermetallic non-equilibrium phases.
The oxidation of reactive intermetallic compounds at comparatively low reaction temperatures has proven particularly valuable for the preparation of intermetallic non-equilibrium phases and has been developed to a versatilely applicable method during the past years. Mostly, solid precursors, e. g. Zintl phases such as Na4Si4 or K4Ge4, are converted with gaseous oxidizing agents. For this purpose, gaseous hydrogen chloride, organic chlorides, ammonia or other suitable gases are applied, depending on the requirements of the chemical system. The reaction principle is depicted in the scheme. Moreover, several other experimental approaches are used, e. g. reactions of dispersed precursors with a dissolved oxidizing agent or the direct conversion of a precursor in a liquid oxidizing agent such as suitably functionalized ionic liquids.
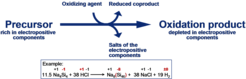
So far, the investigations have focussed on the preparation of intermetallic compounds with silicon and germanium as the framework-forming component. The clathrates obtained by the oxidation method usually distinctly differ in composition from those prepared by other methods, and from the thermodynamic equilibrium state of the respective clathrate phase, which occasionally is known. Furthermore, broad metastable compositional ranges have been accessible, which even may extent to metastable elemental modifications with an emptied clathrate framework. Using such new allotropes as an educt, new metastable intermetallic clathrates have been obtained, which so far have not been accessible by any other preparation method.
Selected literature:
- Arnold M. Guloy, Reiner Ramlau, Zhongjia Tang, Walter Schnelle, Michael Baitinger, Yuri Grin, Nature 443 (2006) 320-323.
- Bodo Böhme, Arnold Guloy, Zhongjia Tang, Walter Schnelle, Ulrich Burkhardt, Michael Baitinger, Yuri Grin, J. Am. Chem. Soc. 129 (2007) 5348-5349.
- Bodo Böhme, Umut Aydemir, Alim Ormeci, Walter Schnelle, Michael Baitinger, Yuri Grin, Sci. Technol. Adv. Mater. 8 (2007) 410-415.
- Bodo Böhme, Stefan Hoffmann, Michael Baitinger, Yuri Grin, Z. Naturforsch. 66b (2011) 230-238.
- Ying Liang, Bodo Böhme, Marianne Reibold, Walter Schnelle, Ulrich Schwarz, Michael Baitinger, Hannes Lichte, Yuri Grin, Inorg. Chem. 50 (2011) 4523-4528.
Acknowledgement:
Sub-projects have been financially supported by the Deutsche Forschungsgemeinschaft (DFG) within the priority program SPP 1415 „Kristalline Nichtgleichgewichtsphasen“ and the priority program SPP 1708 „Materialsynthese nahe Raumtemperatur“, as well as by the European Union (ERDF) and the Free State of Saxony (SAB grant 100112628).
