Heat energy: Any kind of cooling of a hot body involves energy flow to a colder environment. Starting from the first steam engines it has been a major challenge to convert heat flow into a maximum of work. The maximum efficiency (profit vs. costs), which can be only theoretically obtained, is the Carnot-efficiency:
h = (Thot – Tcold)/Thot
Contrary, work can be spent to transport thermal energy. This is the principle of a refrigerator. Thermoelectric heat conversion is a direct energy conversion of heat flow into electrical current. Despite the efficiency thermoelectric generators is limited by Carnot efficiency as well, their real efficiency amounts less than 5% of a combustion engine.
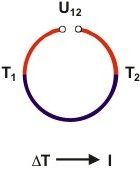
Seebeck effect: An open electrical circuit is built up by two different conducting materials (red and blue) so that there are two junctions. When both junctions are brought to different temperatures (T1 ≠ T2) an electrical current is produced.
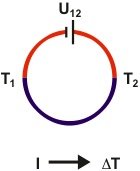
Peltier effect: The circuit is now connected to a power supply. As soon as electrical current flows, both junctions change temperature in opposite way: On is becoming warmer, the other colder.
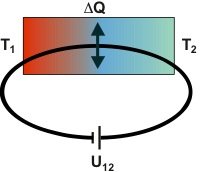
Thomson effect: In this thought experiment, the electrical circuit consists of solely one conductive material and is connected to a power supply. However, the charge carriers have to pass a temperature gradient. In this case, heat flow occurs additional to the normal electrical resistance heating. A copper wire, for example, produces additional heat when the current flows along decreasing temperature and vice versa.


