Chemical vapor transport
Chemical vapour transport (CVT), a technique popularized by Schäfer, is a process where a condensed phase, typically a solid is volatilised in the presence of a gaseous reactant (transport agent) and deposited elsewhere in the form of crystals. Typical transport agents include halogens and halogen compounds. The set-up consists of a 2-zone furnace (source T2 and sink T1), the reactant and transport agent sealed in an ampoule. The various parameters that must be optimised for a successful CVT are growth temperature, transport direction, rate of the mass transport, choice of the transport agent and the free energy of the reaction. Transport is governed by two processes – convection and diffusion. Though larger crystals can be obtained by increasing the transport rates favouring convection, the crystals are inhomogeneous and are prone to having more defects. Thus optimization for each chemical system is vital. Depending on the free energy of the reaction between the species, the source and sink temperature must be altered. A reaction that is exothermic indicates transport from cold to hot zone and the reverse is expected for an endothermic reaction. Also, if the reaction between the species is highly exothermic or endothermic, no transport takes place.
Single crystals of transition metal dichalcogenides (TMD) and pnictides have been obtained by this method.
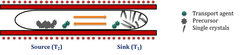
Schematic of the experimental set-up
