Ductile inorganic semiconductor at room temperature
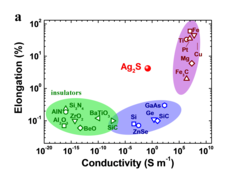
Ductility is a characteristic feature of metals and metallic materials. For semiconductors and insulators, it is rarely observed. Scientists from the Max-Planck-Institut für Chemische Physik fester Stoffe in Dresden and the Shanghai Institute of Ceramics of the Chinese Academy of Science (http://english.sic.cas.cn) report extraordinary metal-like ductility with high plastic deformation strains at room temperature for the semiconductor a-Ag2S which is used in a variety of electronic and optoelectronic devices (Figure 1). This is one of the results of the long-term collaboration between the groups of Profs. Lidong Chen, Xun Shi, and Yuri Grin. The unified picture was obtained by combining the results of different manufacturing and physical characterization techniques with results of quantum chemical calculations. The unusual behavior of the silver sulfide because cleavage along the slip planes is prevented by irregularly distributed bonds of the silver atoms.
Different to the majority of semiconductors and ceramics being usually brittle, α-Ag2S is ductile like a metal and changes shape easily instead of breaking upon deformation. The as-cast ingot obtained by co-melting of Ag and S as well as the sintered specimen manufactured by Spark Plasma Sintering technique exhibit similar densities which are close to the theoretical values. Both bulk samples can be easily machined and thin specimens wind wire-like. Ingots of α-Ag2S can be easily hammered or deformed to a large grade without destroying the material (Figure 2). The microstructure reveals a characteristic feathery pattern (Figure 3).
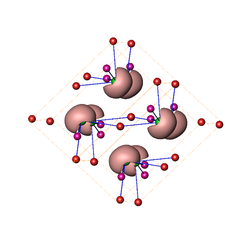
Analysis of the chemical bonding using quantum chemical techniques in position space (QTAIM, ELI) reveals a moderate charge transfer from silver to sulphur and the formation of lone-pairs on sulphur atoms. These are arranged head-to-head along two crystallographic planes with relatively weak atomic interactions. This feature allows for the slipping of the structural units along these planes and – in case of ionic materials – would cause cleavage. This is prevented in α-Ag2S by the irregular bonds formed by part of the silver atoms which move (diffuse) within the structure (Figure 4).
CPfS / JG , UB