A universal strategy to design superior water splitting electrocatalysts with in situ fast surface reconstruction
The development of efficient bifunctional electrodes with extraordinary mass activity and robust stability is a challenging goal for the hydrogen production via water splitting. Surface reconstruction during the electrocatalysis can form fresh-composition electrocatalysts with unusual amorphous phases in situ, which are more active but difficult to prepare by conventional methods. Here, a facile strategy based on fast reconstruction of amorphous nanofilm precursors is proposed to explore precious-metal-free catalysts. As a proof of concept, a SrCo0.85Fe0.1P0.05O3-δ nanofilm precursor with weak chemical bonds deposited onto a conductive nickel foam substrate (SCFP-NF) is synthesized by utilizing a high-energy argon plasma to break the strong chemical bonds in a crystalline SCFP target. The fast reconstructed SCFP-NF bifunctional catalysts show ultrahigh mass activity of up to 1000 mA mg-1 at an overpotential of 550 mV and extremely long operational stability of up to 650 h at 10 mA cm-2, significantly overperforming state-of-the-art precious metal catalysts.
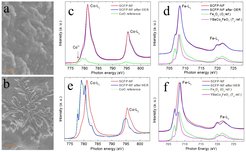
This study, published in Advanced Materials, was perform in a collaboration of researchers from Nanjing Tech University, China; MPI-CPfS, Dresden; Curtin University, Australia; and NSRRC, Taiwan. Dr. Zhiwei Hu, Department of Correlated Matter, investigated the electronic structure of SCFP by x-ray absorption spectroscopy (XAS). The results are shown in Fig. 1(c-f). The valence state of Co and Fe ions at the surface before and after the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) were measured using the surface sensitive total electron yield mode. The Co-L2,3 XAS spectrum reveals that the Co ions in the SCFP (red) has a mixed valence state of +3.18 . The Fe-L2,3 XAS spectrum of SCFP (red) locates at the same energy as that of Fe2O3 indicating a Fe3+ state. The poorly resolved spectral feature of SCFP (red) as compared with that of Fe2O3 (green) suggests the existence of a low local coordination as shown in Figure 1(d) for the YBaCo3FeO7 (magenta) spectrum with a FeO4 tetrahedral coordination. The oxidation states of Co only slightly reduces after OER (blue) in Fig. 1(c), but all Co ions were converted into Co2+ after HER (blue) in Fig. 1(e). The Fe3+ state in SCFP remains unchanged, however, the spectrum becomes slightly sharper after OER (blue) in Fig. 1(d), and much sharper after HER (blue) in Fig. 1(f), where one can see that the t2g and eg related peaks are well resolved similar to that in Fe2O3. This indicates an increase in the coordination number of Fe ions, and in turn an enhanced crystal field interaction of 10 Dq, indicating a surface reconstruction during the electrocatalysis.
These results demonstrate that soft x-ray absorption spectroscopy is a sensitive tool to study the valence state and the local environments of Co and Fe ions before and after electrocatalytical processes, providing a better understanding of the mechanisms causing an improved activity of catalysts.
ZH/CPfS
