New method for imaging electronic orbitals in solids
The research teams of Prof. Liu Hao Tjeng from MPI-CPfS, Prof. Maurits Haverkort from the University of Heidelberg (former MPI), and Dr. Andrea Severing from the University of Cologne have conceptualized and tested a new method for making quantum mechanical objects in solids visible at a beamline at the German Electron Synchrotron DESY in Hamburg.
Orbital states are quantum mechanical constructions that describe the probability to find an electron in an atom, molecule or solid. We know from atomic physics that an s-orbital is spherical or that a p-orbital is dumbbell-shaped, but how do the complicated distributions of the electrons that contribute to chemical bonds in solids look like? Knowledge of these orbital states or electron distributions is the basis for our understanding of chemical bonds and with this of the physical properties; always with the goal to design materials with specific properties. Here x-ray spectroscopy has contributed tremendously but the interpretation of the spectra is not easy and is often based on some assumptions for the analysis of the data. Hence it would be great to have an experimental method that gives a direct image of the local electron density.
Such a method has been developed by the research teams of Prof. Liu Hao Tjeng, Prof. Maurits Haverkort and Dr. Andrea Severing. At the German Electron Synchrotron DESY in Hamburg they have built up an end station that can be used for imaging directly local electron densities without the need for further mathematical analysis. They have shown at the example of Ni2+ in NiO, the textbook example for a strongly correlated material, that the angular dependence of the scattering intensity of the dipole forbidden 3s→3d transition (see Fig. 1) images directly the angular dependence of the Ni2+ 3A2 3d(x2-y2/3z2-r2) orbital (see Fig. 2). This is based on the simple ideal that the 3s orbital is spherical so that its angular distribution does not contribute to the angular distribution of the scattering intensity. The angular intensity of the scattering intensity is therefore a direct image of the angular distribution of the local density of the 3d electrons.
What sounds simple is experimentally not trivial because the transition s→d is dipole forbidden so that it cannot be reached with conventional methods as e.g. x-ray absorption. This transition, however, can be excited with photons when the energy transfer goes along with very large momentum transfers of the order of 10Å-1. These large momentum transfers excite in addition to dipole so-called multipole transitions that have different selection rules so that even 3s→3d transitions have intensity. Such large momentum transfers can be reached is a non-resonant inelastic x-ray scattering experiment, in brief NIXS, with hard x-rays of about 10 keV. The d-orbital can be imaged directly if the initial state is an s-orbital. This method is therefore called s-NIXS.
LHT / CPfS
![Fig. 1: The transition 3s→3d measured at the Ni M1 edge in NiO. The transition is shown for many different directions of the momentum transfer with respect to the sample orientation. These so-called cuts through the single crystal are defined by the directions [001] and [100] in panel (a) and [001] and [110] in the panels (b) and (c).](/2991678/original-1553599155.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjI5OTE2Nzh9--2e8586965ecf7c22a24bb3a1d6a57562009fb41f)
Fig. 1: The transition 3s→3d measured at the Ni M1 edge in NiO. The transition is shown for many different directions of the momentum transfer with respect to the sample orientation. These so-called cuts through the single crystal are defined by the directions [001] and [100] in panel (a) and [001] and [110] in the panels (b) and (c).
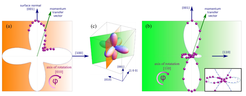
Fig. 2: a, b) Integrated intensities of the M1 transition 3s→3d in Fig. 1 plotted on the respective projections of the 3A2 3d(x2-y2/3z2-r2) orbital of Ni2+. (c) The three dimensional plot of the 3A2 3d(x2-y2/3z2-r2) orbital (more specific: the hole density) with the projections as in (a) and (b), respectively. © MPI CPfS
![Fig. 1: The transition 3s→3d measured at the Ni M1 edge in NiO. The transition is shown for many different directions of the momentum transfer with respect to the sample orientation. These so-called cuts through the single crystal are defined by the directions [001] and [100] in panel (a) and [001] and [110] in the panels (b) and (c). Fig. 1: The transition 3s→3d measured at the Ni M1 edge in NiO. The transition is shown for many different directions of the momentum transfer with respect to the sample orientation. These so-called cuts through the single crystal are defined by the directions [001] and [100] in panel (a) and [001] and [110] in the panels (b) and (c).](/2991678/original-1553599155.jpg?t=eyJ3aWR0aCI6ODQ4LCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6Mjk5MTY3OH0%3D--ca7ac76454015a736da94cb924fdba3aba399cfc)
