Pressemeldung: Metal vacancy orderings in defect anti-perovskite Fe2SeO
In crystalline materials, the ordering of two or more different anions is far from understood. The main reason is probably that Nature (our role-model) cannot perform this chemistry easily, as the different reactants are found spatially separated in the Earth´s crust: Selenides (Se) and oxides (O) have different densities and do not often meet naturally. In the lab, however, we can help them to join.
Perovskites are related to the archetype SrTiO3 crystal structure (Figure). On replacing anions by cations and vice versa, anti-perovskites can form, like Ag3SI that is well-known as Ag+ super-ionic conductor. Neither significant metal deficiency nor possible ordering of the resulting metal vacancies in such compounds was reported.
Through a chemical reaction in a melted salt it was possible to obtain small crystallites of anti-perovskite related Fe2⎕SeO (⎕ = vacancy), where O is found at the center and Se at the corners of a cubic lattice (Figure).
As it happens, Nature orders Fe and ⎕ in two different ways, i.e. α- and β-Fe2SeO modifications, of which one modification was discovered by a visiting undergraduate student.
Although the average crystal structure is directly related to the simple anti-perovskite (Figure), the unique chemical situation results in very complex atomic arrangements. Further, the distribution of Fe and ⎕ in β-Fe2SeO (Figure) has handiness (chirality), which usually only is found in organic systems.
Through collaboration with the ESRF (European Synchrotron Research Facility, Grenoble, France) it was possible to investigate exact purities of powder samples, enabling us to do further investigations of β-Fe2SeO. The magnetic moments on Fe are affected by the rare atomic arrangement; a rare effect that is seen in blood-stone, α-Fe2O3.
The observed Se-O ordering inspires for further explorative investigations and opens up a new window in inorganic chemistry.
MV / CPfS
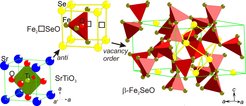
The crystal structure relation from perovskite SrTiO3, via defect anti-perovskite Fe2⎕SeO, to metal vacancy ordered β-Fe2SeO.
