Anionic Open-Shell p Electron Systems
Introduction
The interplay between spin, orbital, charge, and lattice degrees of freedom has been explored widely in strongly correlated transition metal (TM) compounds with partially filled d shells. However, similar phenomena are also observed in open shell p electron systems. For instance, there is some analogy between the properties of TM compounds having Jahn-Teller t2g6eg3 (Cu2+) or t2g3eg1 (Mn4+) electron configurations and alkali metal superoxides AO2 with paramagnetic O2- ions having a degenerate (π*)3 electron configuration (Fig. 1 right). Here, the π* orbitals are antibonding molecular orbitals formed from O 2pπ atomic orbitals. A prototype system is CsO2 where, similar as for the Cu2+ compound KCuF3, a quasi-one-dimensional magnetic ordering was observed, which is driven by orbital ordering. The orbital ordering involves a reorientation of the molecular anions, which is an important degree of freedom in molecular anionic p electron systems.
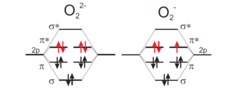
Figure 1: Molecular orbital energy scheme for paramagnetic superoxide (right) and diamagnetic peroxide (left) ions
Recent Results
The main focus of our work is on alkali sesquioxides A4O6 ( A = Rb, Cs) which are anionic mixed valence compounds with two paramagnetic O2- anions and one diamagnetic O22- anion (Fig. 1 left) per formula unit and thus feature also the charge degree of freedom. Surprisingly, the A4O6 compounds adopt a cubic crystal structure (space group I-43d) which only accommodates a single site for the O2n- anions. However, Raman and inelastic neutron experiments revealed the presence of distinct O2- and O22- units. Recently we succeeded to isolate a single crystal of a tetragonal modification of Rb4O6 and to determine its crystal structure (space group I-4) [1]. In this phase charge ordering of distinct O2- and O22- anions is clearly apparent (Fig. 2). As the molecular position and orientation fixes the orbital orientations and lifts the orbital degeneracy it is concluded that charge ordering may be associated with orbital ordering.
![Figure 2: Illustration of the crystal structures of the cubic (left) and tetragonal (right) modification of Rb4O6, taken from Ref. [1]. The blue ellipsoids correspond to Rb+ ions. In the left picture the red dumbbells correspond to the indistinguishable O2- and O22- units, whereas in the right picture the red and yellow dumbbells correspond to O22- and O2- units, respectively](/2872609/original-1518441262.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjI4NzI2MDl9--80dca466ffa344d6eafba58043c60d17dd88bf94)
Figure 2: Illustration of the crystal structures of the cubic (left) and tetragonal (right) modification of Rb4O6, taken from Ref. [1]. The blue ellipsoids correspond to Rb+ ions. In the left picture the red dumbbells correspond to the indistinguishable O2- and O22- units, whereas in the right picture the red and yellow dumbbells correspond to O22- and O2- units, respectively
The formation of a tetragonal modification of A4O6 is also the clue to understand anomalies in the magnetic and structural properties of the sesquioxides, which has been established in depth for Cs4O6. Combining magnetization measurements with EPR and 133Cs NMR measurements performed in collaboration with Denis Arcon, Ljubljana, we have shown that the magnetic properties and structural phases present depend on the chosen temperature protocol [2]. Powder neutron diffraction studies performed in collaboration with Kosmas Prassides (Durham University, UK, and Tohoku University, Sendai, Japan) have shown, that on rapid cooling the ambient temperature cubic phase is frozen, whereas on slow cooling a dominating tetragonal phase, similar to that of tetragonal Rb4O6, is forned. Anomalies seen in the magnetic susceptibility data on heating (Fig. 3) reflect the transformation of the frozen cubic to the tetragonal and the transformation back to the cubic phase, respectively. Our studies provide profound insights into the interplay between spin, orbital, charge, and molecular orientation degrees of freedom in these anionic p electron systems.
![Figure 3: Inverse molar magnetic susceptibility cm-1 of Cs4O6 measured at 1 T as a function of temperature. Anomalies in data obtained on heating (red) are most evident in the product χm ∙T and reflect the structural transformations occurring in samples which were rapidly cooled from room temperature. The figure is taken from Ref [2]](/2872620/original-1518441263.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjI4NzI2MjB9--b12fbc93c990152a0cb64771ca3785e89a340eab)
Figure 3: Inverse molar magnetic susceptibility cm-1 of Cs4O6 measured at 1 T as a function of temperature. Anomalies in data obtained on heating (red) are most evident in the product χm ∙T and reflect the structural transformations occurring in samples which were rapidly cooled from room temperature. The figure is taken from Ref [2]
Deeper insights into the low-dimensional physics of the prototype superoxide CsO2 emerged from recent 133Cs NMR [3] and EPR [4] studies, which were performed in collaboration with Denis Arcon. The one-dimensional magnetic order is confirmed and the analysis of the data suggests that CsO2 can be considered as a rare example for the Tomonaga-Luttinger liquid model. At higher temperatures the dimensionality of the system changes and an unusual variation of the exchange interactions with temperature is found.
Our work on alkali superoxides and sesquioxides has been part of the collaborative EU-Japan project LEMSUPER (Light Element Molecular Superconductivity, 2011 – 2015). It is of interest to compare the properties of these compounds having molecular O2n- units with those of superconducting fullerides containing C60n- units and superconducting rare earth sesquicarbides (Ln2C3) featuring C22- units. In the latter hybridized π*-d states constitute the conduction band, whereas in case of the oxides no metal d states contribute to chemical bonding. Compared to the C60 compounds the oxygen anion based solids are electronically more localized (U >> W, where U is the correlation energy and W the bandwidth). Application of high pressure could be a strategy to metallize these solids and possibly even induce superconductivity. Resistance measurements on Rb4O6 and Cs4O6, which were performed in collaboration with the group of Shimizu and Kagayama at Osaka University, revealed that the compounds stay insulating up to pressures of 80 GPa and 180 GPa, respectively. We have investigated the high pressure structural properties of Cs4O6 and Rb4O6 by synchrotron powder x-ray diffraction and by Raman experiments. Even at pressures of some GPa Cs4O6 transforms completely and Rb4O6 partially to the tetragonal charge ordered phase (Fig.4). Structural changes under pressure may prevent metallization which has been predicted by electronic structure calculations.
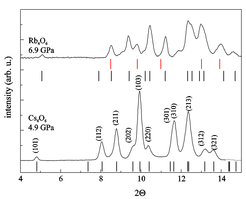
Figure 4: Synchrotron powder diffraction patterns of Cs4O6 and Rb4O6 at the given pressures, which indicate complete (Cs4O6) or partial (Rb4O6) transformation to the tetragonal modification

![Figure 2: Illustration of the crystal structures of the cubic (left) and tetragonal (right) modification of Rb4O6, taken from Ref. [1]. The blue ellipsoids correspond to Rb+ ions. In the left picture the red dumbbells correspond to the indistinguishable O2- and O22- units, whereas in the right picture the red and yellow dumbbells correspond to O22- and O2- units, respectively Figure 2: Illustration of the crystal structures of the cubic (left) and tetragonal (right) modification of Rb4O6, taken from Ref. [1]. The blue ellipsoids correspond to Rb+ ions. In the left picture the red dumbbells correspond to the indistinguishable O2- and O22- units, whereas in the right picture the red and yellow dumbbells correspond to O22- and O2- units, respectively](/2872609/original-1518441262.jpg?t=eyJ3aWR0aCI6MzQxLCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6Mjg3MjYwOX0%3D--607dc8e75bbeeef3296a32643bc5f6c31aac2f5b)
![Figure 3: Inverse molar magnetic susceptibility cm-1 of Cs4O6 measured at 1 T as a function of temperature. Anomalies in data obtained on heating (red) are most evident in the product χm ∙T and reflect the structural transformations occurring in samples which were rapidly cooled from room temperature. The figure is taken from Ref [2] Figure 3: Inverse molar magnetic susceptibility cm-1 of Cs4O6 measured at 1 T as a function of temperature. Anomalies in data obtained on heating (red) are most evident in the product χm ∙T and reflect the structural transformations occurring in samples which were rapidly cooled from room temperature. The figure is taken from Ref [2]](/2872620/original-1518441263.jpg?t=eyJ3aWR0aCI6MzQxLCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6Mjg3MjYyMH0%3D--b8265f0d6075811fac755684cf0f9ebf333e1982)
