News:
Square-planar iridate discovered in the unusual d5 configuration
Physicists and chemists reveal the uncommon properties of a new material, which goes beyond normal chemistry intuition.
Local environments and valence electron counts primarily determine the electronic states and physical properties of transition-metal complexes. For example, square-planar coordination geometries found in transition-metal oxometalates such as cuprates are usually associated with the d8 or d9 electron configuration.
Researchers in our institute address an unusual square-planar single oxoanionic [IrO4]4− species, as observed in Na4IrO4 in which IrIV has a d5 configuration, and characterize the chemical bonding through experiments and by ab initio calculations. A research team including experimental chemists (Martin Jansen, Claudia Felser, and Patrick Merz) and theoretical physicists (Binghai Yan and Sudipta Kanungo) work together and find that the IrIV center in ground-state Na4IrO4 has square-planar coordination geometry because of the weak Coulomb repulsion of the Ir-5d electrons. In contrast, in its 3d counterpart Na4CoO4, the CoIV center is tetrahedrally coordinated because of strong electron correlation. Na4IrO4 may thus serve as a simple yet important example to study the ramifications of Hubbard-type Coulomb interactions on local geometries.
BY / CPfS
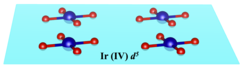
Square-planar iridate discovered in Na4IrO4 with the unusual d5 configuration.












