MnSiPt: Local magnetism rules the chemical bond
Helge Rosner, Andreas Leithe-Jasper, Wilder Carrillo-Cabrera, Walter Schnelle, Sarah V. Ackerbauer, Monika B. Gamza, and Yuri Grin
A crystal structure can be understood as a result of bonding interactions (covalent, ionic, van der Waals, etc.) between the constituting atoms. If the forces caused by these interactions are equilibrated, the so-stabilized crystal structure should have the lowest energy. In such an atomic configuration, additional weaker atomic interactions may further reduce the total energy, influencing the final atomic arrangement.
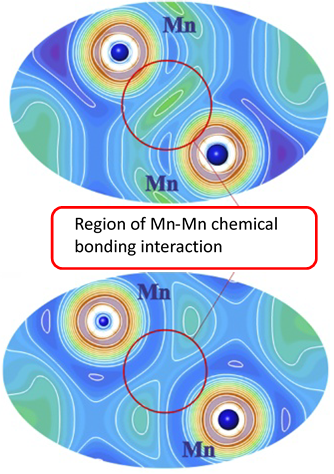
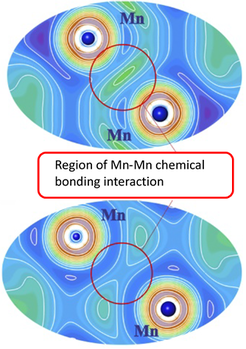
Indeed, by combining a thorough experimental characterization with a theoretical analysis of the chemical bonding of the intermetallic compound MnSiPt, we find that the formation of direct Mn–Mn bonds is suppressed. Surprisingly, the energy gain due to bond formation is significantly smaller than the on-site magnetic interactions. Therefore, after formation of the covalent bonds between Pt and Si as well as between Mn and Si, the strong Mn intra-atomic exchange is the key factor for the stability of the crystal structure. In competition against the Mn–Mn bond formation, intra-atomic magnetic interactions determine the topology of the local atomic arrangement in the finally adopted TiNiSi-type crystal structure in MnSiPt.
![Figure 1. Crystal structure of MnSiPt. Observed crystal structure in the orthorhombic TiNiSi type: The shortest Pt–Si contacts (black bars) form layers of distorted hexagons, which are interconnected along the [100] direction, yielding eight-membered channels, where Mn–Mn zig-zag chains (red bars) are embedded.](/2917143/original-1531312190.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjI5MTcxNDN9--d622c0e6f616d2c94f461da674f0f39b1873879d)
To understand the chemical background of the stabilization of the TiNiSi structure for the MnSiPt compound, an analysis of atomic interactions in real space (i.e., in the language of chemistry) was performed using the Electron Localizability Indicator in its ELI-D representation. The ELI-D drastically illustrates the suppression of covalent Mn–Mn bonding due to the local Mn spin polarization.
ALJ / HR / YG / CPFS
![Figure 1. Crystal structure of MnSiPt. Observed crystal structure in the orthorhombic TiNiSi type: The shortest Pt–Si contacts (black bars) form layers of distorted hexagons, which are interconnected along the [100] direction, yielding eight-membered channels, where Mn–Mn zig-zag chains (red bars) are embedded. Figure 1. Crystal structure of MnSiPt. Observed crystal structure in the orthorhombic TiNiSi type: The shortest Pt–Si contacts (black bars) form layers of distorted hexagons, which are interconnected along the [100] direction, yielding eight-membered channels, where Mn–Mn zig-zag chains (red bars) are embedded.](/2917143/original-1531312190.jpg?t=eyJ3aWR0aCI6ODQ4LCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6MjkxNzE0M30%3D--0e8f404ae7290a0dd1fddb57d6cd6b589fdfd4c9)