c-axis dimer and its electronic breakup: The insulator-to-metal transition in Ti2O3
As a transition metal oxide heats up, it can suddenly change from an insulator to a conductor. This fascinating phenomenon is accompanied by changes in the crystal structure and often also in the magnetic structure. Band structure calculations, the standard theory for describing the electronic structure of solid-state materials, fail to describe this behavior. In particular, band theory has great difficulties to explain how these materials become insulators at low temperature. It’s clear that interactions among electrons must be taken into account, however these types of problems that involve many interacting particles can quickly become unsolvable. It is therefore important in these calculations to have good starting points and smart approximations. We have experimentally identified the key elements of the electronic structure of such a system so that a valid and accurate theoretical model can be constructed.
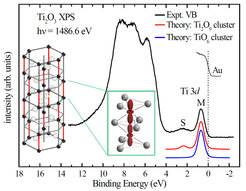
We used X-ray spectroscopies to study the electronic structure of the transition metal oxide Ti2O3. From distinct features in our spectra, we found that the insulating phase can be viewed as a solid consisting of electronically isolated Ti-Ti diatomic molecules (dimers) —contradicting results from band structure calculations. We also see that as temperature increases, the dimers partially break up, which causes the transition toward a metallic state. This is associated with a reconstruction of the electron orbitals that opens up an extra channel for transferring electrons along with a dramatic decrease in the effective Coulomb interaction.
These experiments reveal an amazing amount of detail about all of the relevant ingredients in a metal-insulator transition. These findings may therefore serve as an important benchmark for future theoretical studies.
CPfS / CFC ; LHT