Selective Orbital Imaging of Excited States with X-Ray Spectroscopy: The Example of α-MnS
Transition metal compounds have a wide variety of extraordinary properties, including metal-to-insulator and spin-state transitions, colossal magneto resistance, superconductivity, multiferroicity, and bad metal behavior. To understand these rich phenomena, one needs to look to the wealth of possible electronic states. In particular, one would like to know which electron orbitals are involved. While a variety of techniques exist to probe these states, the complex nature of correlated materials makes analysis of the data far from straightforward. Here, a team of scientists from MPI-CPfS in Dresden, University of Cologne, Heidelberg University, the German Synchrotron in Hamburg (DESY) and the Taiwanese National Synchrotron Radiation Research Center in Hsinchu, demonstrate that it is possible in a spectroscopic experiment to make a direct visual image of the excited states and, by doing so, identify their orbital character [1].
The team carried out experiments on α-MnS, a rock salt type antiferromagnetic insulator with orbital degrees of freedom that are present in its excited states. The newly developed spectroscopic technique is s-NIXS, where large momentum transfers q are used in the scattering of x-rays to access the quadrupolar 3sà3d transition. Spectra were collected for a wide range of q-directions. The directional dependence of the energy-integrated intensity of the spectra provides a spatial image of the orbital occupation in the ground state, as was discovered in an earlier work [2]. What is new here is the study of the directional dependence of the spectral features. It turns out the technique yields also a spatial image of the excited states. This simplifies considerably the identification of the so-called multiplet states which typically dominate the electronic structure of transition metal compounds.

In the α-MnS experiment, the directional dependence of the energy-integrated intensity of the spectra yields a spherical charge distribution, revealing the high-spin 3d5 ground state of the Mn ion. As far as the spectral features are concerned, two peaks can be seen in the spectra, which can be assigned to eg and t2g orbitals on the basis of their angular shapes (Fig. 1). The energy distance between the two peaks (0.78eV) measures the crystal field splitting 10Dq.
The information on the excited states must be interpreted in a many body framework, which takes into account both the full atomic multiplet theory (electron-electron interactions) and the local effect of the lattice. The present study shows that, thanks to the spin-only nature of the 3s hole, one can easily relate the energy splitting between the s-NIXS peaks to the 3d multiplet scheme in absence of the 3s hole. This constitutes a simplification of the analysis since such a multiplet scheme is well studied in the form of Sugano-Tanabe-Kamimura diagrams, see Fig.2.
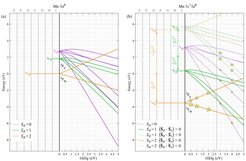
The possible s-NIXS final states are indicated with stars in Fig. 2(b). For 10Dq values on the left of the gray vertical line, the initial 3d5 configuration is the high-spin 6A1, and on the right the low-spin 2T2. The size of each star is proportional to the intensity of the excitation, averaged over all directions. For the high-spin initial state, there are only two states in the diagram that can be reached with the s-NIXS excitation, namely the 5T2 (the extra 3d electron occupying t2g orbitals) and 5E (the extra electron in the eg). From the shape of the final state orbitals as imaged in Fig. 1 (bottom panels), one can identify immediately that the lower energy peak belongs to the 5T2 state and the higher to the 5E. It is then straightforward to understand, that their energy separation of 0.78 eV corresponds one to one to the 10Dq value.
It is worth looking into Fig. 2(b) in more detail. For 10Dq values on the right-hand side of the gray vertical line, the ground state will no longer be high-spin but low-spin. The consequence for the s-NIXS spectrum is dramatic. It switches from a two-peak structure (two stars) into a five-peak feature (five stars). This demonstrates that the line shape of the s-NIXS spectrum is an extremely sensitive indicator of the ground state symmetry. The ground state hole density will also change: the t2g5-like orbital occupation will be highly non-spherical, which can be revealed directly by the image obtained from the directional dependence of the energy-integrated s-NIXS intensity.
s-NIXS spectroscopy is therefore a new powerful technique to probe the orbital physics in transition metal compounds. The present study [1] demonstrates that one can extract from the analysis of s-NIXS spectra (1) the character of the excited states, (2) the relevant energy parameters, and (3) the character of the ground state. In addition, it was shown in a previous study [2] that the integral over the s-NIXS signal also gives (4) direct information about the character of the ground state. Information (4) can then be used to check the information obtained from (3). Alternatively, in case the spectra are extremely complex, information (4) can be used as a constraint for the analysis of the spectra so that (1) and (2) can be extracted more reliably. Therefore, s-NIXS opens up new opportunities to determine the local electronic structure in a wide range of transition metal compounds.
[1] A. Amorese, B. Leedahl, M. Sundermann, H. Gretarsson, Z. Hu, H.-J. Lin, C. T. Chen, M. Schmidt, H. Borrmann, Yu. Grin, A. Severing, M. W. Haverkort, and L. H. Tjeng, Phys. Rev. X 11, 011002 (2021), https://doi.org/10.1103/PhysRevX.11.011002
[2] H. Yavaş, M. Sundermann, K. Chen, A. Amorese, A. Severing, H. Gretarsson, M.W. Haverkort, L.H. Tjeng, Nature Physics 15, 559 (2019), https://doi.org/10.1038/s41567-019-0471-2.












